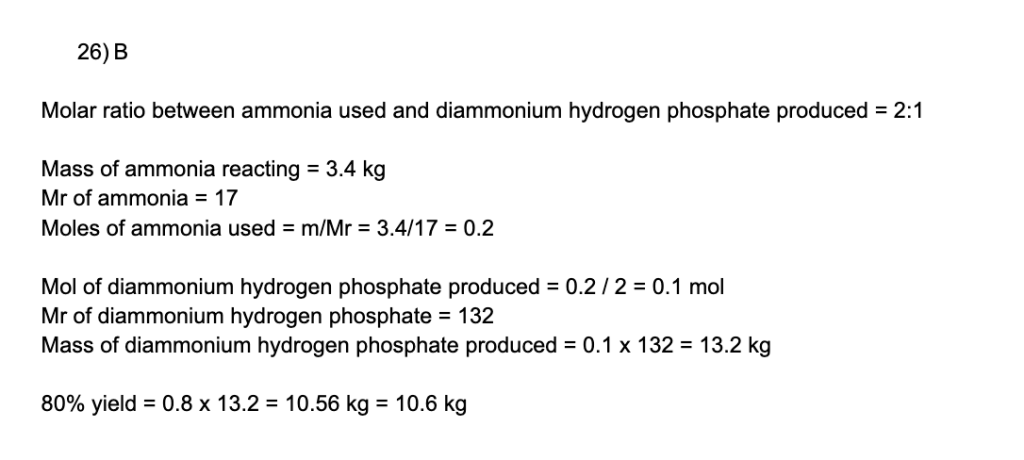
Diammonium hydrogen phosphate, (NH4)2HPO4, can be used as a fertiliser. The following equation shows how it can be synthesised:
2NH3(g) + H3PO4(aq) → (NH4)2HPO4(s)
What is the mass of diammonium hydrogen phosphate that is produced when 3.40 kg of ammonia is reacted with phosphoric acid (H3PO4), in excess, with a yield of 80%?
(Mr values: NH3 = 17.0; H3PO4 = 98.0; (NH4)2HPO4 = 132)
Answer: