Listed are the electronic configurations for the atoms of different elements. Which one represents the most reactive non-metal?
Answer:
5) C
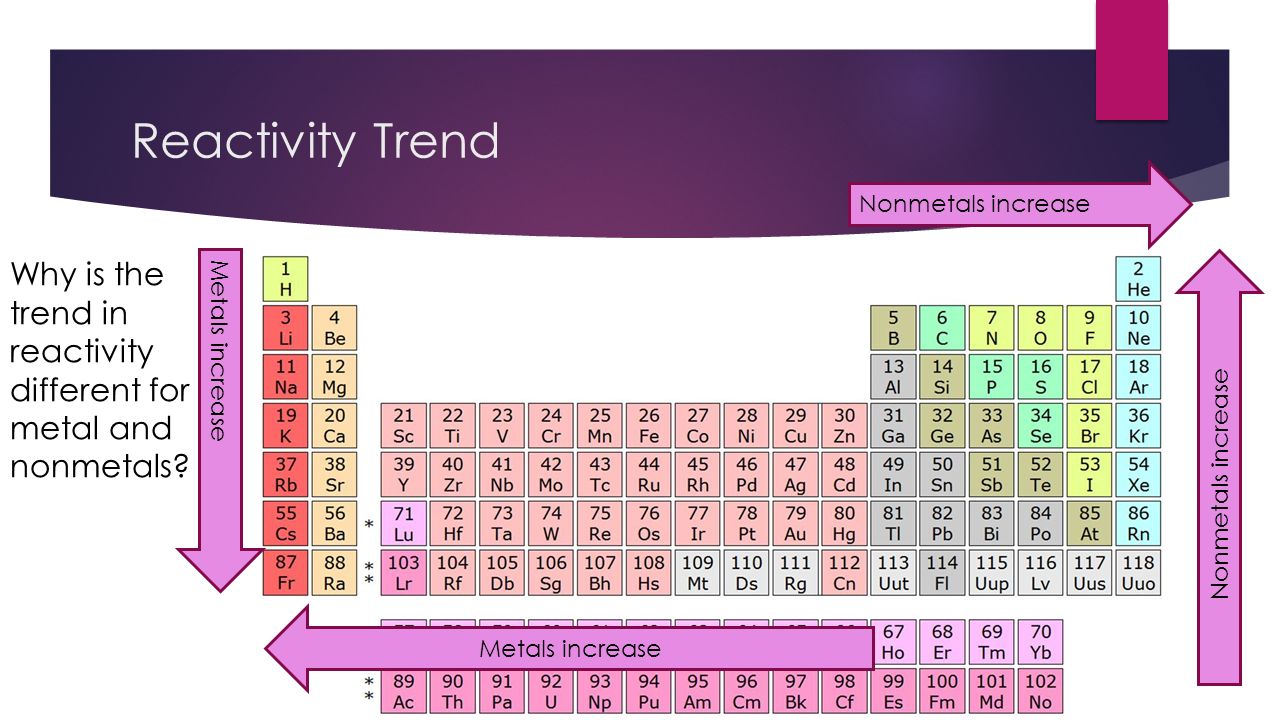
As you go along a period, reactivity of non-metal increases since their electronegativity increases. Therefore, the halogens are the most reactive non-metals within each period.
Noble Gases ARE NOT the most reactive since they are inert.
As you can see from the periodic table below, reactivity increases across a period and up a group for non-metals. Therefore, Fluorine is the most reactive non-metal.
The electronic configuration for Fluorine is 2,7. Hence, C is the answer